The spectral lines of this series corresponds to the transition of an electron from a higher energy state to the 4th orbit. The second step attack on the carbocation electrophile by the hydroxide nucleophile and formation of the new carbon-oxygen bond is a highly exergonic step that passes through a second much lower transition state TS2.
35 Label The Energy Diagram Labels For Your Ideas
For this series p 4 and n 5 6 7.

Labelled energy diagram. Labelled Energy Level Diagram Chemistry Written By JupiterZ Sunday February 4 2018 Add Comment Edit. Energy Level Diagram For Molecular Orbitals Chemical Bonding And. Draw a labelled energy level diagram for an.
Chemistry Electron Orbitals And Sub Levels. Each elementary step has its own activated complex labeled AC 1 and AC 2. School Emily Carr Secondary School.
The wave numbers and the wavelengths of the spectral lines constituting the Brackett series are given by barv1lambdaR142-1n2 iii. On an energy profile the enthalpy change for the reaction is measured from the energy of the reactants to the energy. Energy level diagrams Energy level diagrams are used to model energy changes during reactions.
Diagram Of Pelton Turbine Main Parts Renewables First The. 12 7 Catalysis Chemistry. Learn vocabulary terms and more with flashcards games and other study tools.
Wind Power Next Cc. If playback doesnt begin shortly try restarting your device. Energy conservation is an important concept when analyzing open channel flows.
From the CK-12 Foundation Christopher Auyeung. The Energy Transition Germany S Turning Point Florence School. How To Draw Label Enthalpy Diagrams Video Lesson Transcript.
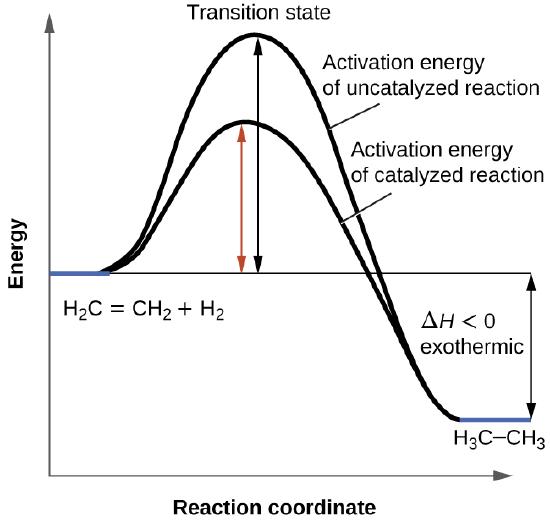
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Bbc gcse bitesize energy level diagrams energy level diagrams during a chemical reaction energy is either taken in or given out during exothermic reactions energy is given out this means that the energy of the products will be lower than the energy of the reactants this can be represented using an energy level diagram potential energy diagrams ap chemistry a potential energy diagram plots the change in potential in the reaction pathway and is labeled at the peak of the activation energy. The activation energy for each step is labeled E a1 and E a2.
Igcse Chemistry 4 14 Represent Exothermic And. Wind Turbine Diagram Images Stock Photos Vectors. The activated complex having the highest potential energy is positioned between the reactants and products.
The main difference in energy band diagram of solid insulator and that of conductor is in case of insulator there is wide energy gap between valance band and conduction band whereas this gap is small in case of semiconductor. The reaction is exothermic so the potential energy of the products should be lower than the potential. Diagram shows that the reactants have higher potential energy than the products.
Start studying Labeling an Energy Diagram. This preview shows page 16 - 18 out of 28 pages. If light of wavelength 5000 oA is incident on the surface of the metal find the threshold frequency and incident frequency.
In 1913 Neil Bohr proposed a theory to explain the stability of an atom where electrons revolved around the nucleus at certain energy levels. Labelled Wind Energy Diagram Written By JupiterZ Thursday March 16 2017 Add Comment Edit. Draw a labelled energy profile diagram.
This enthalpy diagram has starting products ending products delta H and activation energy labeled. How to draw an energy level diagram. Pages 28 Ratings 67 3 2 out of 3 people found this document helpful.
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. They show the relative energy levels of the products and reactants. An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram.
However this does not happen and Rutherfords model could not explain why the collapse does not occur. Course Title BIOLOGY 12U Biolog. Labeled Energy Diagram UNTPIKAPPS Rena Porter Heat pump diagram.
It also shows the effect of a catalyst on the f. For the purposes of the following analysis energy is conserved for a fluid in an open channel flow and head losses due to friction will be neglected. Please log in or register to add a comment.
This series lies in the ne ar infrared region of the spectrum and contains infinite number of lines. Represented with the help of diagram called energy band diagram. The transition state for this step labeled TS1 can be thought of as that point at which the carbon-chlorine bond is halfway broken.
Bohrs Model of the Atom. Draw a neat and labelled energy level diagram and explain Balmer series and Brackett series of spectral lines for hydrogen atomThe work function for a metal surface is 22 eV. Energy Diagram Labeled.
Draw a labelled energy level diagram for an endergonic reaction Label the. A complete enthalpy diagram will include starting energy ending energy and E a and delta H. Check Your Solution Check that the labels are all included.
In an energy diagram. The loss of energy would ultimately slow down the electron speed and finally the electron would fall into the nucleus. Enthalpy change ΔH is the amount of energy absorbed or released by a chemical reaction.
Wind Turbine Wind Power Going Through 7th Grade. The reaction whose potential energy diagram is shown in the figure is a two-step reaction. Draw a Neat Labelled Energy Profile Diagram for Sn1 Reaction Mechanism.
Activation Energy And The Activated Complex Energy. The energy of the activated complex.
The activation energy for each step is labeled E a1 and E a2.

Labelled activation energy diagram. The activation energy is shown as a hump in the line which. The reaction whose potential energy diagram is shown in the figure is a two-step reaction. For the purposes of the following analysis energy is conserved for a fluid in an open channel flow and head losses due to friction will be neglected.
The activation energy is the difference between the energy of the reactants and the maximum energy ie. Draw a horizontal line from the highest part of the curve towards the vertical axis. Catalysts speed up the rate of reaction by lowering the activation energy.
For this reason the activation energy of a reaction is sometimes referred to as the activation energy barrier. In an energy diagram the vertical axis represents the overall energy of the reactants while the horizontal axis is the reaction coordinate tracing from left to right the progress of the reaction from starting compounds to final products. Activation Energy Diagram Labeled Written By JupiterZ Wednesday September 2 2020 Add Comment Edit.
Label the energy diagram for a two step reaction. In the s n 1 reaction the carbocation species is a reaction intermediate. A potential energy diagram for an s n 1 reaction shows that the carbocation.
The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. Each elementary step has its own activated complex labeled ac 1 and ac 2. AE reactants activation energy Energy transition state activated complex products Reaction progress Question.
Energy Diagram Labeled. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. This enthalpy diagram has starting products ending products delta H and activation energy labeled There are two different types of energy changes in.
Label this energy diagram. Starts at the energy of the reactants. Potential Energy Diagram Labeled Inspirational Important Questions.
The activation energy for each step is labeled e a1 and e a2. Draw a labelled energy level diagram for an endergonic reaction Label the from BIOLOGY 12U BIOLOG at Emily Carr Secondary School. The activation energy for each step is labeled e a1 and e a2.
Energy conservation is an important concept when analyzing open channel flows. On an Energy Profile the activation energy is measured from the energy of the reactants to the peak of the energy profile diagram. Potential energy diagrams ap chemistry a potential energy diagram plots the change in potential in the reaction pathway and is labeled at the peak of the activation energy Energy Level Diagram In A Sequence Diagram New In A Sequence Diagram Unique Sequences potential energy diagram how to label and use a potential energy diagram.
Labelled Energy Level Diagram Chemistry Written By JupiterZ Sunday February 4 2018 Add Comment Edit. The reaction between textH_2 textg and textF_2 textg Figure 124 needs energy in order to proceed and this is the activation energy. The lower the activation energy the faster the reaction will proceed.
Each elementary step has its own activated complex labeled AC 1 and AC 2. Activation energy diagram labeled. These are some keyword suggestions for the term Activation Energy Graph Labelled.
Activation Energy Diagram. Endo and exothermic reactions Exothermic reaction. Activation energy graph labelled activation energy graph labeled Latest updated pages.
Label the energy diagram for a two step reaction. Energy Level Diagram For Molecular Orbitals Chemical Bonding And. Draw and label the activation energy.
Potential Energy Diagrams How to draw and label PE diagrams for exothermic and endothermic reactions and what effect adding a catalyst or inhibitor has on the diagram. Energy conservation is an important concept when analyzing open channel flows. Labeling Parts Of A Reaction Coordinate Diagram Youtube.
Activation energy is an integral part of understanding kinetics of chemical reactions. Endothermic because energy is needed to break the a b bond. Label this energy diagram.
Is equal to the difference in energy between the top of the hump and the reactant. The activation energy E_a is the difference between the energy of the activated complex and that of the reactants. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams.
It also shows the effect of a catalyst on the forward and reverse activation energy. Chemistry Electron Orbitals And Sub Levels.
By default electrons are found in the lowest energy level possible close. Draw and label the activation energy.
Igcse Chemistry 2017 3 5c Draw And Explain Energy Level Diagrams To Represent Exothermic And Endothermic Reactions
When an excited electron returns to a lower level it loses an exact amount of energy by emitting a photon.

How to draw energy level diagram. Draw the energy level diagram. At energy level 2 there are both s and p orbitals. Draw a horizontal line from the highest part of the curve towards the vertical axis.
305 Triple only draw and explain energy level diagrams to. Draw arrows to show the E a and Δ H including their values Worked example. Where Z is the atomic number and n is the energy level.
The three dashes in 2p subshells represent the same energy. In order to find the energy of the photon that was absorbed or emitted you always take the higher energy level and subtract from it the lower energy level. Therefore the order of energy level is as follows.
In an energy diagram the vertical axis represents the overall energy of the reactants while the horizontal axis is the reaction coordinate tracing from left to right the progress of the reaction. 304 calculate the molar enthalpy change ΔH from the heat energy change Q. The energies needed to remove inner shell electrons do not significantly change from the energy levels of isolated atoms.
1The sub shell of a particular shell do not have equal energiesFor Ex. 303 calculate the heat energy change from a measured temperature change using the expression Q mcΔT. Energy level diagrams are used to model energy changes during reactions.
The Lymanultraviolet series of spectral lines corresponds to electron transitions from higher energy levels to level. Zoomed in version of the energy level diagram. σ1s.
The first ten molecular orbitals may be arranged in order of energy as follow. There must be a hump in the curve to represent the energy level of the activated complex. 2s and 2p have different energies.
Energy Levels Orbital Diagrams Electron Config Noble Gas - Google Slides. The 2s has lower energy when compared to 2p. Determining the activation energy.
Draw and label two short horizontal lines to mark the energies of the reactants and products. So in this case we would take -6eV and subtract from it -10eV which tells us that it would take a four eV photon to bump an electron up to that energy level and the electron would emit a four eV photon if it dropped back down from that level. Ive never done it for a publication but for diagrams I still have a tendency to use ChemDraw.
An energy level diagram. They show the relative energy levels of the products and reactants. Note that in equation 3 there are two moles of phosphorus.
For hydrogen the ionization energy 136eV. The periodic table shows us energy levels 1 7. I did it for a spectroscopy class where we had to draw diagrams for every little thing.
Use a C-C bond for each level you can get parallel lines etc. 4s has lower energy when compared to 3d. The ground state is represented by n 1 first excited state by n 2 second excited state by n 3 etc.
4 Ps Cl 2 g PCl 5 s ΔH -463 kJ. Below is a blank energy level diagram which helps you depict electrons for any specific atom. Instead they are meant as a rough illustration of the effect.
The energy change must be adjusted to show the transformation for 1 mole of phosphorus. The amount of energy required to rip off an electron is represented on the energy level diagram by the vertical distance from that level to the ground level at the top of the diagram. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams.
Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important observations from energy level diagrams of multi electron atoms are. It shows the energy in the reactants and products and the difference in energy between them.
The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n. Draw the curve in the energy level diagram clearly showing the transition state Step 4. The enthalpy level diagram can now be constructed.
Shows whether a reaction is exothermic. We know atoms have energy levels. Using the Bohr Model the energy levels in electron volts eV are calculated with the formula.
Energy level diagram for Molecular orbitals. 2In a particular shell sub shell. En -136 Z2n2 eV.
