Advertisement Remove all ads. Then electrolytic refining of copper is carried out to remove the rest of the impurities present in the ore.
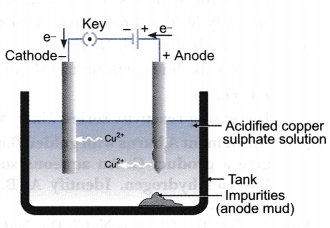
What Is Meant By Refining Of Metals Describe The Electrolytic Refining Of Copper With A Neat Labelled Diagram Cbse Class 10 Science Learn Cbse Forum
CBSE Class X Science SA 2.

Draw labelled diagram of electrolytic refining of copper. This process is known as roasting. Concept Notes Videos 538. Asked Mar 21 2020 in Chemistry by Sandhya01 591k points metals and nonmetals.
At the anode Cu - 2e----- Cu 2 The impure block of copper thus will be used The impurities settles at the bottom of the electrolytic cell. I First step is the heating of sulphide ore of copper in the presence of an excess of air. Copper from Cu 2 S can be obtained from its ore simply by heating in air.
Firstly the apparatus for the electrolysis consist of Electrolytic tank containing acidified copper sulphate solution as. Draw Neat Labelled Diagram of Electrolytic Refining of Blis Ter Copper. On passing the current as shown in the diagram the pure copper from the anode dissolves into an electrolyte.
What is meant by refining of metals draw the diagram of electrolytic refining of copper and name the substances used as cathode anode and the electrolyte share with your friends share 12 refining of metals most metals obtained by the reduction process are not very pure electrolytic refining of copper refining is the final step. Refining copper diagram with labels Refining Copper Diagram With Labels Class 10 science chapter 3 ncert exemplar solution partiiiclass 10 science chapter 3 ncert exemplar solution partiiiMay 12 2017 iii electrolytic refining b draw a neat and well labeled diagram for electrolytic refining of copper ans a b the diagram showing the electrolytic refining of copper refining copper diagram with. In the purification of copper by electrolytic refining the strip of impure copper metal is made the anode and a strip of pure copper metal is the cathode.
In this setup an electrolyte metal salt aqueous solution depending on the metal is often used. Describe electrolytic refining of copper with chemical equations. This method is widely used as purification of metals like zinc Zn copper Cu aluminium Al chromium Cr tin Sn lead Pb nickel Ni gold Au.
Electrolytic refining is a process of refining a metal mainly copper by the process of electrolysis. I First step is the heating of sulphide ore of copper Copper from Cu 2 S can be obtained from its ore simply by heating in air. A solution of copper sulphate is used as the electrolyte.
Draw a well labelled diagram for it. During electrolytic refining of zinc it gets a deposited on cathode. So they can displace copper from copper.
The crude copper serves as anode while a thin sheet of pure copper serves as cathode. Refining improves the. Process Of electrolytic Refining is described below.
Copper from Cu 2 S can be obtained from its ore simply by heating in air. Asked Apr 12 2020 in Chemistry by Mukesh01 476k points metals and nonmetals. Asked Jul 9 2018 in.
Draw a well labelled diagram for it. Then electrolytic refining of copper is carried out to remove the rest of the impurities present in the ore The impure copper is made anode and strip of pure copper are made cathode On passing the current as shown in the diagram the pure copper from the anode dissolves into an electrolyte An equivalent amount of copper is deposited on the. In this process impure metal is used as anode a strip of pure metal is used as cathode and soluble salt of metal is used as electrolyte.
An equivalent amount of pure copper from the electrolyte is deposited on the cathode. Applying suitable voltage to the electrodes causes oxidation of copper metal at the anode Cu - 2e Cu2 and reduction of Cu2 to form copper metal at. This is known as anode mud or slime.
Draw Neat Labelled Diagram of Electrolytic Refining. As far as the mechanism of the process is concerned during electrolysis a large chunk or slab of impure metal is used as the anode with a thin strip of pure metal at the cathode. Draw the diagram of the apparatus used in electrolytic refining of copper and label the electrode where pure copper is deposited.
Electrolytic Refining of copper means its refining by electrolysis method. Copper is purified upto 9995 purity using electrolytic refining method An electrolytic cell is prepared to do this refining Diagram shown below is an electrolytic cell used for copper refining In such a cell a thin sheet of highpurity Cu serves as the cathode and the anode is the impure Cu which is to be refined. Soluble impurities remain in the solution whereas.
The impurities iron zinc etc ionize and dissolves in the copper sulphate solution. Draw labelled diagram for the electrolytic refining of copper. An equivalent amount of copper is deposited on the cathode.
Draw labelled diagram for the electrolytic refining of copper. Draw a well labelled diagram for it. The electrolyte consists of an acidic solution of CuSO4.
The impure copper is made anode and strip of pure copper are made cathode. Question Bank Solutions 12104. Electrolysis of copper from copper sulphate solution electrolyte is done using copper electrodes.
On passing electric. Draw labelled diagram for the electrolytic refining of. II The second step is the self-reduction of copper.
Maharashtra State Board HSC Science Electronics 12th Board Exam Question Papers 164. When current is passed through the electrolyte pure copper from the anode dissolves in the electrolyte. Many metals like copper zinc tin lead are refined by this method.
Draw a neat diagram of Electrolytic refining of copper and label the parts. They are above copper in the activity series. Find an answer to your question Describe electrolytic refuting of copper with chemical equations.
The copper anode itself loses electrons and gives copper ion in the solution.
NCERT P Bahadur IIT-JEE. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless.
How Electrolytic Refining Of Copper Is Carried Out Explain In Detail Sarthaks Econnect Largest Online Education Community
Draw a Neat Well Labelled Diagram of Electrolytic Cell for Extraction of Aluminium - Chemistry.

Draw a neat and well labelled diagram for electrolytic refining of copper. Electricity is passed through solutions containing copper compounds such as copperII sulphate. Electrolysis Copper is purified by electrolysis. Click hereto get an answer to your question Draw a neat and well - labelled diagram for electrolytic refining of copper.
A very thin copper plate is electro-plated with gold III using gold chloride in HCI. Draw a neat and well labelled diagram for electrolytic refining of copper - Science - Control and Coordination. 1 M and E o Z n 2 Z n 0.
NCERT P Bahadur IIT. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Draw a neat diagram of Electrolytic refining of copper and label the parts.
To keep watching this video solution for FREE Download our App. Copper is purified by electrolysis. View solution Calculate the half cell potential at 2 9 8 K for the reactoin Zn 2 2 e Zn if Zn 2 0.
Draw a neat labelled diagram of electrolytic cell for the extraction of aluminium. Step by step solution by experts to help you in doubt. Draw neat and labelled diagram of Besemer converter used in the extraction of copper.
Draw a neat and labelled diagram Electrolytic reduction of alumina Draw a neat and labelled diagram Electrolytic reduction of alumina Books. Draw a neat labelled diagram of electrolytic cell for the extraction of aluminium. Draw a neat and labelled diagram Electrolytic reduction of alumina.
Getting Image Please Wait. CopperII oxide carbon copper carbon dioxide 2CuO C 2Cu CO2 Removing oxygen from a substance is called reduction. The current was passed for 20 min.
And the increase in the weight of the plate was found to be 2g. Draw neat and labelled diagram of Besemer converter used in the extraction of copper. Draw a neat and labelled diagram Electrolytic reduction of alumina.
In this process impure metal is used as anode a strip of pure metal is used as cathode and soluble salt of metal is used as electrolyte. On passing electric current through the electrolyte cations move towards. Join the 2 Crores Student community now.
Draw a neat and labelled diagram Electrolytic reduction of alumina. Draw a neat diagram of Electrolytic refining of copper and label the parts. Draw a neat and labelled diagram Electrolytic reduction of alumina.
Draw a neat and labelled diagram Electrolytic reduction of alumina Apne doubts clear karein ab Whatsapp par bhi. Draw a neat and well - labelled diagram for electrolytic. Watch 1000 concepts tricky questions.
Electricity is passed through solutions containing copper compounds such as copperII sulfate. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. The anode positive electrode is made from impure copper.
352 k 130 k Answer. Draw a neat labelled diagram of electrolytic cell for the extraction of aluminium. This method is widely used as purification of metals like zinc Zn copper Cu aluminium Al chromium Cr tin Sn lead Pb nickel Ni and gold AuIn this process impure metal is used as anode a strip of pure metal is used as cathode and soluble salt of metal is used as electrolyte.
Draw the diagram of the apparatus used in electrolytic refining of copper and label the electrode where pure copper is deposited. Draw a neat labelled diagram of electrolytic cell for the extraction of aluminium. Zinc is more reactive metal as compared to copper therefore zinc displaces copper from coper sulphate solution.
- Sarthaks eConnect Largest Online Education Community. The copper oxide is reduced to copper in the reaction above. This method is widely used as purification of metals like zinc Zn copper Cu aluminium Al chromium Cr tin Sn lead Pb nickel Ni gold Au.
Open Answer in App. Loading DoubtNut Solution for you.